Biology (SPBR)
Stechnolock: Plant Biology and Research
Full Text
Volume 1, Issue 1
Survey and Management of Cabbage Aphid (Brevicoryne brassicae L.) on Cabbage Using Botanical Extracts under Irrigation Condition at Dera District, North West, Ethiopia
*Corresponding Author: Alula MK, South Gondar Zone Agriculture and Livestock Development Department, Debere Tabor, Ethiopia, Tel: +251918739417, E-mail: ledatemare@gmail.com
doi: /spbr.2021.1.104
Citation: Alula MK, Tesfaye A (2021) Survey and Management of Cabbage Aphid (Brevicoryne brassicae L.) on Cabbage Using Botanical Extracts under Irrigation Condition at Dera District, North West, Ethiopia. Stechnolock Plant Biol Res 1:1-23
Copyright: © 2021 Alula MK. This is an open-access article distributed under the terms of Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cabbage is very important vegetable crop in Ethiopia and cabbage aphid is also one of the key insect pest of cabbage which causes severe yield losses of the crop. The objective of this study were to assess the status of cabbage aphids infestation, damage and evaluate the efficacy of the locally available botanicals for the management of cabbage aphid at Dera district, North west, Ethiopia. A Cross-sectional study design was conducted to assess cabbage aphid infestation status on cabbage. The experiment was laid out in a Randomized Complete Block Design (RCBD). The data was collected through interview questionnaire survey; farmer field visit and field experiment using botanicals for the management of cabbage aphid. Data was entered; analyzed using SAS version 9.0. The result of the respondents showed that most important insect pests of cabbage production were cabbage aphid (68%). The main control measures use were synthetic insecticides (78%) and the remain uses other methods (19%) like the urine of cattle, Besana (Croton macrostachya) and dega avalo (Combretum molle).The prevalence of cabbage aphid on farmer filed visit was (43%). In fields’ experiments obtained results revealed that all tested materials were exhibited mortality rate action against cabbage aphids. Efficacy percentage of botanical reveals that Azadirachta indica (48%) followed by Phytolacca dodecandra (44.6%) which were significantly superior in efficacy against cabbage aphids after three days of first spray application. A significantly higher mean yield in ton per hectare 46.86±3.75 and 50.074±2.60 were recorded at Azadirachta indica and Phytolacca dodecandra treated plots respectively. The highest yield loss protection and marginal return rate were noted too. As a conclusive, Azadirachta indica and Phytolacca dodecandra could be suggested as an alternative management option of Brevicoryne brassicae (L.) for smallholder farmers.
Keywords: Botanical, Brevicoryne brassicae, Cabbage, management
Introduction
Background and Justification
Cabbage (Brassica oleracea Var.Capitata) is grown on 3.1 million hectares (ha) of land in the world. In Africa, the precise quan- titative data is not available but in some countries show huge production of cabbages. For instance; in Kenya, estimated annual production is 550,000 tones, with 95% of production in highlands on 35000 ha (FAO, 2007). In East Africa, about 90% of the Brassica production is by smallholder growers on plots of 0.1-0.5 ha (Grzywacz et al., 2010) [1].
Cabbage (Brassica oleracea) is the second most important vegetable crop in Ethiopia with respect to production next to red pepper (Capsicum spp) (Tesdeke and Gashawbeza, 2014) [2]. The land occupied for cabbage during 2015 was 35,927.13 ha with a produc- tion level of 355,679.895 tons annually (CSA, 2015) [3].
Cabbage (Brassica oleracea) is a cruciferous vegetable which is a very important crop for smallholder farmers providing income and nutrition and enabling small farmers to remain financially viable, especially in rapidly growing pre-urban farming sectors (Grzywacz et al., 2010) [4]. Cabbage has high nutritional value and source of food for humans and animals. It consists of water (92.8%), protein (1.4 mg), calcium (55.0 mg) and iron (0.8 mg) in 100g cabbage; the leaves are eaten raw in salads or cooked (Cervoni, 2017) [5]. Ethiopia has the favorable agro-climatic conditions for the production of head cabbage for fresh market (Tesdeke and Gashawbeza, 2014) [6]. However, cabbage yield and quality is influenced by several factors, among of these biotic (i. e., diseases, insect pests, and weeds) and abiotic factors (i.e., soil acidity and low soil fertility) as well as institutional (e.g. policy, market, and infrastructure). The high incidence of insect pest and diseases infestation further accentuates high pre-harvest and postharvest losses. Among in- sect pests, cabbage aphid (Brevicoryne brassicae L. Hemiptera: Aphididae) is very serious in cabbage growing areas of this country especially in the dry season (Grzywacz, et al., 2010 and Shibru and Negeri, 2016) [7,8].
Cabbage aphid causes significant yield losses in many crops in the family Brassicaceae, which includes the mustards and crucifers. Continued feeding by aphids causes yellowing, wilting and stunting of cabbage (Opfer and Mcgrath, 2013) [9]. Severely infested plants become covered with a mass of small sticky aphids, which can eventually lead to leaf death and decay. Cabbage aphids feed on the underside of the leaves and on the center of the cabbage head (Griffin and Williamson, 2012) [10]. They prefer feeding on young leaves and flowers and often go deep into the heads of cabbage. It also secretes honeydew while feeding on leaves that reduce the quality of cabbage. With the development of sooty mold on this honeydew, the product has become sooty and unmar- ketable (Hines and Hutchison, 2013) [11]. Aphids also transfer dangerous viral and bacterial diseases. At sever infestation, the yield may decrease by 34-62% (Afonin et al., 2008) [12].
Controlling cabbage aphid is not an easy practice although synthetic pesticides are apparently available for use. Effective pest con- trol is no longer a matter of heavy application of limited insecticides, because continuous use of pesticides promotes development of insecticidal resistance in the target pests, pest resurgence, emergence of secondary pests, affects non-target insects’ species, affects the environment and human health (Sarwar, 2015 and Grzywacz et al., 2010) [13,14].
Therefore the use of alternatives including botanicals, bio-pesticides, and new generation synthetic insecticides is essential to growing healthy crops of cabbage. The use of different botanical insecticides to protect plants from pests is very promising because of several distinct advantages (Marbet and Aurea, 2008) [15]. Insecticidal plants are generally much safer than conventionally used synthetic insecticides. It prevents development of insecticide resistance. Botanical insecticides are used by small-scale farmers at low cost or zero cost of extraction and are highly degradable. The use of natural and easily biodegradable crop protection inputs like Neem (Azadirachta indica); Kitkita (Dodonaea angustifolia); Garlic (Allium sativum) Ginger (Zingiber officinale) and Endod (Phy- tolacca dodecandra) can be a useful component of an integrated pest management (IPM) strategy since the compounds are known for their low toxicity against beneficial insects (Koona and Njoya, 2004) [16]. These botanical plants are the commonly found in this North Western region of Ethiopia. There was limited information about botanical management of cabbage aphid in spite of its high prevalence in the Amhara Region North west Ethiopia. Therefore, the aim of this research was to show effects of some botanical on cabbage aphid management and head cabbage production and productivity in the study area.
Materials and Methods
Description of Study Area
Location: The study was conducted at Dera district during 2017/2018 off season (October to February). It is located about 607 km North West from Addis Ababa and 47 km from Bahir Dar town the capital city of Amhara Region. Dera district is one of the districts in South Gondar Administrative Zone, Amhara National Regional State, Ethiopia (Figure 1) (South Gondar Department of Agriculture Annual Report, 2012) [17].
Geography and Topography: Dera district is bordered to the south by the Abay river which separates it from western Gojjam ad- ministrative zone, in the west it is bordered by Lake Tana, in the north by Fogera district, and in the east by Estie district. It covers a total area of 158,948 ha, of which 35% is plain, 20% is mountainous, 18% is gorges and 27% is undulating. The altitude of the district ranges from 1,500 - 2,600 m above sea level (Ashagrie et al., 2014) [18].
Climate or Weather conditions: As to the agro-ecology, 85% is Woynadega (subtropical/1500-2300 meter above sea level.) while 15% is Dega (2300-3200 meter above sea level). The annual average rainfall is 1,250 mm. The average minimum and maximum temperature of this study area were 180c and 300c respectively (Molla et al., 2005, Ashagrie et al., 2014) [19,20].
Farming systems and livelihood: The total population of the district is 259,113 of which 132,367 are male and 126,746 are female. The number of households in the district is 51,129 of which 45,757 are male headed and 5,370 are female headed. The farming system of the study areas is characterized by crop-livestock mixed farming. Farm households depend mainly on crop and livestock both for food and cash income. Four types of cropping season are known in the study areas, namely main season (rain-fed agricul- ture), residual moisture, full irrigation in the dry season and recession farming. The major crops cultivated in the district are maize, millet, teff, rice, chick pea (residual moisture); grass pea (residual moisture) under rain-fed conditions. The principal crops grown under irrigation are tomato, shallot, head cabbage, maize, barley, potato, garlic, fenugreek, pepper, emmer wheat and bread wheat. Chick pea and grass pea are also produced using residual moisture and either double cropping or relay cropping (Molla et al., 2005, Ashagrie et al., 2014) [21,22].
Part I
Survey Methodology
Sampling, Sample Size Determination and Assessment
Information of insect pests on cabbage aphids; farmers’ aphid management practices and other valuable information were col- lected by interview with structured and semi-structured questionnaires. Surveys were carried out in Dera district; in five kebele Administrations that are Zara, Korata, Tebabari, Jigna, and Shemie where cabbage production is takes place respectively (Figure 1). The kebeles were selected by purposive sampling based on accessibility and logistic considerations to carry out the study. In each Kebele, the village leaders were asked to generate a list of cabbage cultivating farmers. A questionnaire was designed for 30 cabbage producer farmers from five representative kebeles. A total of 150 cabbage-growing households were interviewed during study period.
Socio-economic characteristics of cabbage producers, cabbage production practice and its constraints, Factors affecting cabbage aphid (increased cabbage aphid and decreased cabbage aphid), aphid managements used by farmers, opinions and requests about cabbage production was collected using questioner. Through the questionnaire information were obtained about socio-economics characteristics were sex, educational status, size of holdings, land ownership, type of land acquisition, cabbage agronomic practic- es / number of times cabbage is cultivated in a year, inputs used/ and farmers’ perception about cabbage aphid, perceived yield loss and pest management strategies in use. The questionnaires were interviewed by Kebele development agents (DAs). Additional information was gathered by field visits.
Assessment and Sampling of Farmer Field
Five kebeles were selected for cabbage aphid field assessment and evaluation. There was an average of 75 cabbage producers per kebele in Dera district from which 5 were sampled. In each kebele these five cabbage producing farmers were selected by random sampling. Each farm selected for the assessment was divided into four quadrants and from each part four plants were selected at random within the square meter. A total of 80 cabbage plants were observed in a single farm at maturity stages during the study period.
In field visit the status of cabbage aphids, distribution of cabbage aphids, and their natural enemies in the study area were ob- served. That is the number of aphids per plant were assessed. For other pests the types of pests occur; number of pests per plant and number of plants affected were observed. The plants were sampled by observation for the presences of cabbage aphid and other insect species were also taken into consideration (Adamu et al., 2000). The aphid pest status were determined by the num- ber of aphid tagged on leaves were counted with the help of hand lens and mean number of aphid per plant were calculated using formula. The per cent plant infestation was worked out with the help of the following formula (Koul, 1998).
Part II. Field Experiment Part
Effects of Botanicals on Cabbage Aphid Description of the Study Area for Field Experiment
The field experiment was conducted using irrigation at Zara kebele Administration (KA) during 2017/2018 off season (October to February). Zara kebele is located about 10 km North West from the district capital town Ambesamie. The total area covered by the kebele is estimated to be 2,129 ha, of which 1,102 ha is arable land, 523 ha is grazing land and 504 ha is forest and town. The topography of the kebele comprises 91% plain, and 9% undulating. The number of households in the kebele is estimated to be 2,749 of which 2700 are male headed and 749 are female headed. The minimum and maximum temperatures of the kebele are 19oC and 30oC, respectively, and the average rain fall ranges 1,000 mm and 1200 mm, respectively (Molla et al., 2005, Ashagrie et al., 2014) [24,25].
Experimental Materials
- Cabbage seeds (Copenhagen Market variety)
- Fertilizer (di-ammonium phosphate (DAP) and urea)
- Botanical extracts with rate of concentration as depicted below (Table 1).
Experimental Design and Treatments
The experiment was laid out in a Randomized Complete Block Design (RCBD) with seven treatments and replicated three times. Land slope were used as a blocking factor. 4.5m x 1.5m plots were prepared and 0.5 m pathway was left between plots and 1.5 m was left between blocks. Each plot was having crop rows at spacing of 0.45m in-row and 0.50m inter row (AFFRA, 2013 and Baidoo et al., 2012) [26,27]. The average totals of 30 plants per plot were planted.
The head cabbage (B. oleracea var. capitata) variety was used for the experiment. Seedlings was grown on raised seed bed and transplanted when seedling reached third to fourth true leaf stage. The cabbage was irrigated uniformly. Plots were fertilized with di-ammonium phosphate (DAP) and urea at the rate of 200 and 100 kg/ha, respectively (AFFR, 2013) [26]. The whole amount of DAP were applied just before transplanting, while urea were applied by splitting the total amount in two. Half of the urea was applied one month after transplanting and the remaining half at the beginning of head formation stage. Other field management practices like weeding, cultivation and maintenance of ridges was carried out as needed at the same time for all treatments.
Data Collection and Sampling Technique
There were two data collections; questioner and head cabbage field assessment (survey data) field experimental data. Field experi- mental scouting was start one week after transplanting and was carried out for seven successive weeks. The experimental fields were scouted every week for the signs and symptoms of aphids’ damage and occurrence level. Sampling was done by systematic random sampling and was used on selecting plants and the k-factor of 5 was used. A total of six plants per plot were sampled. Numbers of aphid per cabbage head were counted using hand lens. After treatment application numbers of live cabbage aphids’ post-treatment data was recorded after 3 days of each spray. Finally, the cabbage head yield was measured and was expressed as kg/plot.
Experimental Procedure
Treatments Preparations and Application
Five botanical extracts; local check/ untreated check and standard check insecticide/ Dimethoate (1.5 l/ha) were applied as treat- ments. Applications of treatments were started three weeks after transplanting. Treatments were applied weekly until about fifteen days before harvest. The botanical suspensions were sprayed on the cabbage aphid populations using hand sprayer at the rate of 150 liters per hectare. There was one time spray per week interval. Botanicals were prepared one day before treatment application following the respective procedure described below.
- Aqueous Ginger rhizome Preparation: Fresh ginger rhizomes (Boziab variety) were bought from market and ground using manual grinder. Ground ginger of 250 g was added into 500 ml of distilled water and allowed to stand for 24 hours. The infusion was later filtered using a 40 mm muslin cloth the aqueous juice was labeled and kept in room temperature until it was used. At the time of application the preparation was diluted in 3 L of water and stirred well before spraying (Amuji et al., 2012) [28].
- Aqueous Garlic bulb Preparation: The scale of matured garlic bulb (Beshoftu netch variety) was peeled off and 200 g of peeled clove was put in 1 L of water and ground with a blender to obtain garlic juice. The juice was thoroughly mixed with additional 1 L of water. The mixture was then sieved to obtain a uniform juice and kept in the room temperature until used (Nayem and Rokib, 2013) [29].
- Aqueous EndodBerry Preparation: The matured berries (local variety) was washed with tap water and cut into small pieces. These pieces were dried under shade at room temperature (25oc) till they completely dry. The dried berries were ground with mortar and pestle. The powders were dissolved in distilled water at the rate of (50 g/L water). The solutions were allowed to stand for 24 hours and then the mixtures were filtered through filter cloth for spraying on field (Amuji et al., 2012) [30].
- Aqueous Neem kernel Preparation: Neem preparation (Azadirachta indica local variety); The Neem juice were prepared by grinding dry Neem seeds using mortar and pestle, and sieved using wire mesh. The juice was made by mixing the powder with water in plastic container at the rate of 50g powder per litter of water. After mixing, the solution was stirred carefully until all the powder was mixed completely with the water. This solution was left overnight. The following morning the juice was filtered into the sprayer using plastic mesh for field use (Sarwar, 2015) [31].
- Aqueous Kitkita leaves Preparation: Kitkita (Dodonaea angustifolia) local variety - extract 100g leaves were crashed and then added to one liter of hot water. The extracts were filtered into the sprayer using plastic mesh for field use (Sarwar, 2015) [31].
Estimation of Aphid Population and Treatment Efficacy Percentage
Averages of six plants per plot were sampled. Totally 126 plants were selected randomly and examined for the presence of aphid. The numbers of aphid tagged on leaves were counted with the help of hand lens and mean number per plant were calculated. The numbers of aphids were recorded before spray and after 72hrs hours after application of botanical or chemical at weekly interval thereafter. The number of aphids per plants recorded then calculates the efficacy of each treatment by using the following efficacy percentage formula (Abbott, 1987) [32].
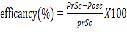
Where: PrSC=Pre Spray Count
PoSC=Post Spray Count
Field Evaluation of Botanicals
Canopy Spread
Canopy spread were measured with a ruler at the time of harvest. The spread of canopy was measured as the horizontal distance from one end of the plant to the other i.e. the two most outspread and directly opposite leaves of the plant was measured using centimeter (Baidoo, 2012) [33].
Plant Height
Plant height was measured from the soil surface to the apex of the plant using centimeter at the time of harvest. The highest point
reached by the plant was recorded as the height of the plant (Asare et al., 2010) [34].
Stand Count
Stand count at crop establishment (on 21/11/2017) and at harvest (on 05/03/2018) was under taken by counting the number of plants in each plot. Number reduction in plant stand was calculated as a difference between stand counted at establishment of seedlings and harvest.
Cabbage Head Formation
Cabbage head formations in each plot were recorded during harvesting. Total number of cabbage plants with head and without
head were recorded separately.
Yield
Marketable and unmarketable yield data were taken from each plot, by removing the outer damaged leaves and discarding heads with less than 4 cm in diameter. Colonies of aphids, development of sooty mold /become sooty on cabbage are unmarketable (Hines and Hutchison, 2013) [35]. The marketable cabbage was measured using kilogram per hectare.
Relative Yield loss Minimize due to Treatment
Yield loss minimized due to treatment was estimated by comparing the yield of treated cabbage with the untreated control (Asare
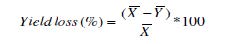
Where x̄ =the mean cabbage yield of treated plots
ȳ = the mean cabbage yield of untreated plots
Economic Analysis
To assess the benefits derived from the application of each treatment, the simple partial budget technique was employed as de- scribed by CIMMYT (1988). Marginal analysis concerned with the process of making choice between alternative factor-product combinations considering small changes. Marginal rate of return is a criterion which measures the effect of additional capital in- vested on net returns using new managements compared with the previous. To measure the increase in net return associated with each additional unit of cost (marginal cost), the marginal rate of return (MRR) was calculated as
MRR = ∆NI/∆IC
Where, MRR is marginal rate of returns, ∆NI – change in net income compared with control, ∆IC – change in input cost compared
with control.
Gross field benefit: it was computed by multiplying farm gate price that farmers receive for the crop when they sell it.
Total cost: It includes the material and the application costs. The cost of garlic, ginger and Dimethoate 40% EC was considered. Collection cost of Neem kernel, endode berry and Kitkita leaves also considered. These prices based on 2017 off- season market. The cost of inputs and production practices such as labor cost for land preparation, weeding, hoeing, watering and harvesting were assumed to remain the same among all the treatments. Price of cabbage per kg and total sale from each plot was also considered. On untreated plot there only inputs (seed and fertilizer) and production practice cost which was the same for all treatments.
Net benefit: was calculated by subtracting the total costs from the gross field benefit for each treatment.
Data Analysis
The survey part data were entered and analyzed using statistical software SPSS version 20 for descriptive statistics (IBM Corp, 2011) [36]. The occurrence of cabbage aphid and some descriptive statistics were analyzed by this software. Data analysis for field exper- iment part was carried out using the SAS version 9.2. After efficacy percentage was calculated the value was subjected to Analyses of variance (ANOVA) to know the mean effects of botanicals and chemicals on aphid mortality. Means were compared using least significant difference and mean separation was done by LSD. To stabilize the variance count data was transformed to logarithmic scale and percentage data was transformed to square root scale (SAS Institute, 2005) [37]. The mean value of the recorded agro- nomic data’s was also subjected to analysis of variance (ANOVA) (Gomez, 2011) [38]. If there was significant difference among the treatments, mean separation was carried out using significance difference at P <0.05.
Results and Discussions
Survey of Cabbage Aphid Infestation, Damage and Farmer Management Practice Socio-economic/Demographic Characteristics of Respondents
A total of 150 farmers were involved in the study. Most of the house heads in the study area are male-headed 74% and the reaming 26% were females. The reason is due to heavy work in irrigation practice and the presence of thief that females unable to keep at maturation hence males are more engaged in cabbage production than female in irrigation of the area (Ashagrie et al., 2014) [39]. The educational levels of respondents were low which were 58% cannot read and write educational level; 38% had no formal education but can read write-only; and only 4% having primary education. Approximately 72% had been farming of cabbage for less than five years, 6.67% had been farming of cabbage for more than five years but less than 10 years while 21.33% have been cultivating cabbage for more than 10 years (Table 1).
Respondent Farmers of Cabbage Farming
Respondents’ farm characteristics are presented in Table 1. Size of holdings, land ownership, type of land acquisition and the number of times cabbage is cultivated in a year are given prominence. Generally, farmers covered in the survey were small-scale farmers. Land use for cabbage were 7%, 89% and 4% of farmers use >0.02Ha, 0.01-0.02Ha and < 0.01Ha, respectively. Majority of respondents, about 96% cropped cabbage once per year and the remaining 4% twice (Korata and Zara) Kebeles within a year.
The study area is obviously the less household cabbage production area (0.01-0.02Ha) compared to the country cabbage average area which is about 0.012 ha as indicated by CSA (2015). Different from our findings study conducted by Kidanie et al. (2016) [40] in Lay Armachiho district the average size of land used for cabbage production by household heads were 0.036 hectares which were higher than our study area. The lowest household’s cabbage production area and produce once per year incurred in our study area might be due to the low level of education. The level of education may influence the adoption of new crop production technology including cabbage. This can be expressed in the low level of production and productivity of cabbage which was observed in this study. In line to this, Oyekale and Idjesa, (2009) [41] reported that extremely low level of education affects the level of technology adoption and skills among farmers.
Cabbage Production Practices and Challenges
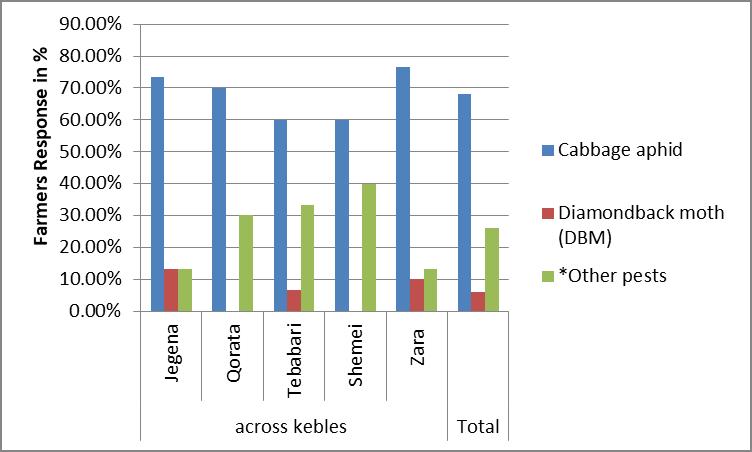
In all villages, farmers commonly raised their own cabbage seedlings from seed bought from retailer shops in Bahir Dar town and Hamusit cooperatives. All farmers expressed that the seed variety they use were Copenhagen variety. The most important prob- lems related with cabbage production reported in the district were the presence of disease and insect, unavailable of the selected seed of cabbage, unavailable of market demand and sustainable availability of water for irrigation. The most important insect and disease of cabbage production as farmers described were the overall averages of cabbage aphid 68%, diamondback moth (DBM) (Plutella xylostella L. Lepidoptera: Plutellidae) 6% and other pests 26% (flea beetles (Phylloterta spp), wild animal, rat, termite etc...). Cabbage aphid was expressed more frequently in Zara and jigna Kebeles than other Kebeles (Figure 1).
Cabbage production and cabbage aphid seasonal calendar
The peak season of cabbage aphid occurs in the area was February to April 56.67% of farmers respond while the remaining 26% explains November to January, and 17.33% others depicts May to July. No one express August to October (Table 2 and Figure 2). The reason for aphid flourish might be that appropriate temperature and humidity for cabbage aphid’s population build up appears at February to April. Different scholars depicted the temperature dependency and positive role on aphid densities of cabbage aphid (Aheer et al. 2008, Pal and Singh, 2012; Atsebeha et al., 2009) [42-44]. Similar to our finding study conducted by Aheer et al. (2008) [42] in Russia reported that peak population of aphid species occurred during March when temperature rise from 7.7°C to 25°C. He reported that temperature had a significant and positive role on aphid densities while relative humidity had a negative and significant correlation with aphid population growth. Another scholars in line with our finding reports independently maxi- mum population build up and flight activity occurred during February and March in both seasons when temperature reach 16-37°C (Atsebeha et al., 2009, Pal and Singh, 2012) [43,44].
Factors like dry/sunny weather, less water in cabbage plots and continuous cabbage cropping increase/ flourish cabbage aphid. Production of cabbage using irrigation lacks mostly enough moisture which probably intensifies the occurrence of the most com- mon insect pest of cabbage namely aphids (Fereres et al., 1988) [45]. On the other hand wet/rainy weather, pesticides effectively used, and crop rotation might decrease aphid infestation in cabbage crop.
Seventy percent farmers in the study district were said that dry/sunny weather has increase the aphid population in cabbage crop. The remains 23.33% expressed that insecticides incorrectly used increases infestation of the crop by aphid. This might be due to the development of resistance of aphids for the given insecticides (Sarwar, 2015; Pal and Singh, 2013; Ahmad and Akhtar, 2013) [46-48]. On the other 71.33% supposed that hand wet/rainy weather decreases aphid population in their cabbage farm. The re- mains 20.67% and 8% believed the application of insecticides and crop rotation practiced are decrease cabbage aphid respectively (Table 2). No one respondent was stated the other agronomic practice such as weeding, intercropping, fertilizer applications, spacing of cabbage; repeat plowing etc…has effect on cabbage aphid population. This might be due to un-aware of small holder farmers in the area that indicates inadequate extension by Development Agents (DAs) about these agronomic practice effects on cabbage aphid pests.
Management of Cabbage aphid
The methods used by farmers to manage cabbage aphid in Dera district were the use of pesticides (78%), urine of cattle (8.67%), leaves of Bessana (Croton macrostachya) (6.67%) and leaves of Dega Avalo (Combretum molle) (3.66%) and no intervention (3%) in their descending order Figure 3.
The reason for those farmers who have no intervention in control of aphid in their farm were due to the cost of pesticide is high and they also explained there is no other method of intervention other than pesticides. This finding might be indicates that these small holder farmers lack of advice on non-synthetic methods of pest control methods. Similar to this finding study conducted in central rift valley region of Ethiopia, by Mengistie et al., (2017) [49] indicates that small holder farmer’s lack of advice on integrated pest management and non-synthetic methods of pest control methods hence if not gain pesticides they do not intervene.
Approximately 80% of respondents were aware of the negative effects of using synthetic pesticides in controlling aphids. According to farmers saying prominent side effects included, itching eyes, running nose and skin rashes. They also explain affects non-target insects like honey bee and insecticidal resistance. That is farmers reported at least one symptom of acute pesticide poisoning and chronic effects of pesticide on environment and development of resistance. The reason for acute pesticide effects might be due to inappropriate personal protective wearing during spray that might be due to lack of knowledge. Study conducted by Mengistie et al., (2017) in Central Ethiopia indicates farmers’ level of knowledge of pesticide safety is insufficient.
Similarly, different scholar’s reports continuous use of pesticides promotes the development of insecticidal resistance in the target pests, pest resurgence, the emergence of secondary pests, affects non-target insects’ species, affects the environment and human health (Sarwar, 2015 and Grzywacz et al., 2010, Pal and Singh, 2013; Ahmad and Akhtar, 2013) [50-53]. In spite of pesticides nega- tive effect, they explain the indiscriminate use of pesticides in our study area might be due to the low level of attitude, ignorance of effect on humans and environment and not knowing other management options.
Lastly, the cabbage farmers were asked for whatever opinion(s) they would like to give on cabbage production. Most of the farmers were responded that they want better market opportunities, improved cabbage variety with disease and insect resistance, as well as training in cabbage production and pest management.
Assessment of Farmer’s Field
Farmer Field Visit and Cabbage Aphid Status
The highest cabbage aphid infestation (aphid/plant) was observed at Jigena Kebele Administration that is 445 and the lower at Zara Kebele Administration which was 337 mean numbers of aphids per plant. Other pest infestation rates relatively the highest number 3 was recorded in Shemie kebele and followed by Korata kebele 2 but was no pest number recorded in other Kebele administrations (Table 3). Similar to our finding study conducted in Pakistan by Ali et al., (2015) [54] showed that in highly infested cabbages the number of aphids were high up to 435 per plant. Another study in line with our findings conducted by Nagappan, (2012) [55] in Gondar Zuria district found 413 to 840 aphids per plant.
Plant Infestation Percent in Farmers Field
In 25 farmer field 400 cabbages were assessed for presence of cabbage aphid of which 43 % were infested by aphid pest (Figure 4). The highest cabbage aphid percent plant infestation (68%) was recorded in Korata kebele. It was followed by Tebabari, Jigena, Zara and Shemie, which had 45%, 37 %, 36% and 29% average percent plant infestation, respectively. Rel- atively the lowest percent plant infestation occurrence was observed in Shemie (29%).
Differently to our findings study conducted by Kidanie et al, (2016) [56] head cabbage (Brassica oleracea var. capitata) under irri- gation conditions in Lay Armachiho district, north west Ethiopia showed that aphids, cut worms and flea beetles were among the major insect pests observed in the area. The highest aphid percent plant infestation (100%) was observed of the sample farms. The variability of aphid percent plant infestation between Kebeles of our study area and other study depicted above might be due to variability of agro climatic condition (Atsebeha et al., 2009, Pal and Singh, 2012).
Effect of Botanicals on Cabbage and Aphid at Field Condition Effect of Botanicals against Cabbage Aphid
The fields’ experiments result showed that mean percent reduction of aphid population reveals, among all the treatments. Di- methoate 40% EC, Neem kernel, and Endod berry (82%, 48% and 44.6%) were recorded to be significant (p<0.05) superior in efficacy against cabbage aphids of within three days of first spray application, respectively compared with control. All botanicals gave the high mean percent reduction of aphids (Table 4). However, among the treatments Ginger rhizome, Kitkita leaves and Garlic bulb gave moderate mortality rate (24%, 20% and 19%) compared with the control (Untreated) treatment which is (15.66%)
respectively.
This high efficacy percentage of botanical treated plots in this study might be due to the nature of botanicals that has significant fa- tal effects, antifeedant with significantly lower feeding rates and repellent effects against insects when their populations are low to moderate in size. Other scholars support this finding; the most potent bioactive compounds responsible for insecticide properties in botanicals are alkaloids, non-proteic amino acids, steroids, phenols, flavonoids, glycosids, glucosinolates, quinones, tanins, ter- penoids, salanine, meliantrol, azadiractin, piretrolone, cinerolone and jasmolone acting as contact poisons, ingestion or stomach poisons, feeding deterrences, repellents and confusants, leading to finally death of the insect victim (Sarwar, 2015, Khater, 2011) . The results in this study were similar with other scholar’s findings in different times. For example, Amuji et al., 2012, express that extraction of ginger has showed promising results against cabbage aphid. Study in Zimbabwe shows combinations of garlic and chilli had suppressive influence on insect numbers leading to increased yields. Application of 10g garlic + 10g chili performed best in terms of reduction of aphid population and also increasing yield (Pahla et al., 2014) [57].
In Ethiopia different scholars try to depicted different botanical aphid managements options in different time that has in line with our finding; For instance study conducted by Shiberu and Negeri, (2016) [58] shows Extracts of neem (Azadirachta indica) seed, Kitiketa (Dodonae angustifolia) fresh leaf and Lemon grass (Cymbopogon citrates) gave positive performance under laboratory while the efficacy percent were decline on field trial. Other study conducted at Gondar Ethiopia by Michael and Raja, 2012 [59]; evaluates of Melia azedarach Linn, plant extracts against Cabbage Aphid Brevicoryne brassicae Linn was tested in the field. They found that the repellent activity was significantly greater in leaf discs treated with Melia azedarach Linn at higher concentration. The number of infested cabbage plants and aphid population in the field was decreased significantly in plant extract treated plots.
Nagappan (2012) [60], Study conducted in Gondar Ethiopia also depicted that application of Melia azedarach Linn. (Meliaceae) dry fruit juice applied plot showed significant difference in aphid population compared to control. This study concludes that Melia azedarach can be a suitable alternative method to protect cabbage crop against aphid infestation particularly small farming com- munity those who are unable to afford cost of chemical pesticides. Study conducted in Arsi area, Ethiopia indicates that Neem seed juice used to reduced aphids population by 80.5%. The highest level of Endod concentration tested (10%) had produced cumulative effect of 100% kill (Megersa, 2016) [61].
In this study finding; botanical effects were highly reduced to economic threshold level of aphid population up to third spray es- pecially Neem kernel and Endod berry like Dimethoate 40% (standard check). They make null after fourth spray Table 4. This may be due to their repellent and killing effect after repeated spray pressure and also elates fly away in search of new feeding grounds, leaving the old colony behind. This finding was similar with others, reported that the treated insect with Azadirachta indica extract usually acts primarily as a repellent when applied in the farms (Nagappan, 2012 and Michael and Raja 2012). Megersa, (2016) suggests Endod do have some repellant effect on aphids under field condition.
Effect of Botanicals on Some Agronomic Characteristics of Head Cabbage Effect of Botanicals on Plant Height and Canopy Spread
There was no significant difference (P < 0.05) among treatments in affecting height of plant and canopy spread (Table 5). This is different from other scholar finding those of who indicated that significant difference among treatments in affecting plant height that was cabbage sprayed with neem produced the tallest head cabbage plant in field experiment in central Ethiopia (Begna and Damtew, 2015) [62]. Medium plant height was measured from head cabbages treated with other botanicals (Neem, lantana, gin- ger, garlic). However, control cabbage had the shortest plants height (Asare et al., 2010 and Begna and Damtew, 2015) [63,64].
Effect of botanicals on stand count, head formation and yield
An obvious stand count, head formation and yield increase in Endod berry treated was significantly (P<0.05) higher compared with the control. Endod berry treated followed by Neem was giving higher yield over the control. A significantly higher mean stand count (28.33±0.58), head formation (26±2) and yield (50.074±2.60ton/ha) were recorded at Endod berry treated plot. Which was the highest yield recorded next to standard check / Dimethoate 40% E.C. (52.0±3.90 ton/ha). Relatively lower mean stand count (21±2.65), head formation (16.33±2.52) and 31.59±3.27 yield in ton/ha, were recorded in Kitkita leaves treated plots among bo- tanicals next to control (Table 4.5). An increase in Endod berry treated plots of mean stand count, head formation and yield might be due to that it decreases the parasitic insect of the head cabbage plant so, express its full potential. This finding has analogous with other scholars finding. For instance, Kabir et al, (2014) and Sarwar, (2015) concluded independently that Endod berry botan- icals, showed the superior performance compared with control that sucking pests killed hence growth and yield of the crop was enhanced. Study conducted at Gondar Ethiopia by Michael and Raja (2012) was found that the number of infested cabbage plants and aphid population in the field was decreased significantly in neem treated plots that increases yield of head cabbage. Differently of Our finding study conducted in central parts of Ethiopia by Shiberu and Negeri, (2016) [65] shows of neem (Azadirachta indica) seed, was insignificant difference yields of head cabbage when compared to the control.
Yield Loss and Economic Return of Cabbage as affected by Botanicals
There were significant differences among treatments in reducing yield losses caused by cabbage aphid (Table 6). Total (ground) cost includes the material and the application costs. The cost of garlic and ginger and the cost of Dimethoate 40% EC were based on 2017 off- season market. Neem kernel, Endod berry and Kitkita leaves collection cost also considered. The cost of inputs (seed and fertilizer) and production practices such as labor cost for land preparation, weeding, hoeing, and irrigation and harvesting were assumed to remain the same among all the treatments. On untreated plot there only inputs (seed and fertilizer) and production cost which was the same for all treatments. Net benefit: was calculated by subtracting the total costs from the gross field benefit for each treatment.
Thus the highest level of yield increase percent compared to the control was obtained from Endod berry 42.30% which was similar to standard check Dimethoate 40%. Moreover yield loss protected due to; Neem kernel, Garlic bulb, Ginger rhizome and Kitkita leaves were 31.63%, 25.13%, 24.27% and 21.32%, respectively.
Results of the economic analysis are presented in (Table 6) showed that spraying of cabbage infected with aphid with Endod berry (290325.9 ETB/ha) gave the highest net benefit and with the highest marginal rate of return (37.07%), compared with control. The next highest net benefit from botanicals were Neem kernel (264593 ETB/ha) with marginal rate of return (31.36%). The economic evaluation indicated that controlling cabbage aphid population using botanicals increased net benefit and marginal return rate at least (2.81%) when compared to untreated check. The lowest marginal return rates (1.98%) were recorded in Dimethoate 40% EC (standard check) due to high chemical cost. This finding was similar with other study that reports controlling pest population using botanicals increased net benefit and marginal return rate (Begna and Damtew, 2015) [66]. Nagappan (2012), Study conducted in Gondar Ethiopia also depicted that Melia azedarach is an economical alternative method to protect cabbage crop against aphid infestation particularly small farming community compared to chemical pesticides. Compressive study by Sarwar, (2015) depicted that botanical treatment of pest are cheaper and more accessible in less developed countries so recommended to use by small scale farmers [67-86].
Conclusion and Recommendations
Conclusion
Most of the farmers in the study area were male headed and low educational level. The cabbage producers covered in the survey were small scale farmers with small land area use for head cabbage production. They also produce head cabbage once a year during offseason under irrigation. All farmers expressed that the seed verity they use were Copenhagen market. The most import- ant problems related with head cabbage production reported in the district were presence of insects. This study results indicates that cabbage aphid infestation is high at growth stage of the head cabbage and the main problem in the district. Season was the main factor to flourish cabbage aphids in the study area. Also, aphid flourish time and the farmers cultivating period were coin- cide so as need of intervention to control. To control this insect most head cabbage producing farmers abuse the use of synthetic insecticides. The use of synthetic insecticides for controlling of insect pests may have problems. Of these, for sound management of insects there was an increasing interest in biotic control using plant products, which can prove eco-friendly with highly reduced negative effects on environment. As a conclusive remark, all botanicals tested (Endod berry, Neem kernel, Kitkita leaves, Ginger rhizome and Garlic bulb) had been proved to have capacity of killing cabbage aphids under field condition. Endod berry followedby Neem kernel were found relatively the highest effective botanicals in causing significantly high rate of mortality of aphids com- pared to control. Moreover, in addition to field efficacy of these botanicals had high yield, high economic return rate and minimiz- ing of yield loses due to aphid were recorded.
Recommendations
Based on the above findings the following recommendations were suggested:
The farmers should reduce use of chemical insecticides and rather implementation of botanical insect management strategy to enhance environmentally safe and sustainable production of cabbages. This would ensure high production, maximum protection for humans, domestic animals and wild life
To boost head cabbage production botanical has aphid killing effect on field trial so farmers have an option to use the locally available treatments especially Endod berry and Neem kernel for suppressing cabbage aphid infestation.
Training of development agents, commercial vegetable growers and farmers about the use of botanicals and side effects of chemical insecticide suggested to be given in the study district
Further study of other botanicals Besana (Croton macrostachya) and Dega avalo (Combretum molle) in the district and the above studied botanicals; dose, extraction procedure and mode of action are recommended.
Use of oil extracts as botanical work and some additives for botanical synergetic effects on pest should be done for the future
The study of public health and environmental consequence resulting from the misuse of pesticides is also suggested
- Abbott WS (1987) A method of computing the effectiveness of an insecticide. Bureau of Entomology, United States Department of Agriculture. Journal of the American mosquito control association 3: 302-3.
- Adamu RA, Dike MC, Akpa AD (2000) Preliminary studies on insect pests of green gram (Vigna radiate L Wilezek) in Northern Guinea Savanna of Nigeria. In Entomology in Nation Building: The Nigerian Experience, Nigeria.
- Agriculture, Forestry and Fisheries Republic of South Africa (AFFRA) (2013) Production guide line in cabbage, South Africa.
- Afonin AN, Greene SL, Dzyubenko NI, Frolov AN (2008) Interactive Agricultural Ecological Atlas of Russia and Neighboring Countries. Economic Plants and their Diseases, Pests and Weeds, Russia.
- Aheer GM, Ali A, Munir M (2008) Abiotic factors effect on population fluctuation of alate aphids in wheat. J Agric Res 46: 367-71.
- Ahmad M, Akhtar S (2013) Development of insecticide resistance in field populations of Brevicoryne brassicae (Hemiptera: Aphididae) in Pakistan. Journal of Economic Entomology 106: 954-8.
- Ali KI, Ahmad M, Akbar R, Sajjad H, Muhammad S, et al. (2015) A study on losses due to Brevicoryne brassicae in different Brassica genotypes under screen house conditions. Journal of Entomology and Zoology Studies; 3(6): 16-19.
- Amhara Regional Agricultural Research Institute (ARARI), 2005. Horticultural crops production training manual for Developmental Agents volume 3. 5-19. Unpublished
- Amuji CF, Echezona BC, Dialoke SA (2012) Extraction fractions of ginger (Zingiber officinale Roscoe) and residue in the control of field and storage pests. Journal of Agricultural Technology 8: 202-3.
- Asare E, Addo A, Mohammed A (2010) Control of Diamondback Moth (Plutella xylostella) On Cabbage (Brassica oleracea Var Capitata) Using Intercropping with Non-Host Crops American. Journal of Food Technology 5: 269-72.
- Ashagrie A, Ayana M, Tefera T (2014) The Global Water Initiative Programme in Dera Woreda, South Gondar Zone of Amara National Regional State. Global Water Initiative - East Africa Secure water for smallholder Agriculture, Ethiopia.
- Atsebeha S, Alemu T, Azerfegne F, Addis T (2009) Population dynamics of aphid and incidence of Ethiopian pepper mottle virus in central rift valley. Crop Protection 28: 443-8.
- Baidoo PK, Adam JI (2012) The effects of extracts of Lantana camara (L.) and Azadirachta indica (A. Juss) on the population dynamics of Plutella xylostella, Brevicoryne brassicae and Hellula undalis on cabbage. Sustainable Agriculture Research 1: 229-34.
- Baidoo PK, Mochiah MB, Apusiga K (2012) Onion as a Pest Control Intercrop in Organic Cabbage (Brassica oleracea) Production System in Ghana. Sustainable Agriculture Research 1: 1.
- Begna F, Damtew T (2015) Evaluation of four botanical insecticides against Diamondback Moth, Plutella Xylostella L. (Lepidoptera: Plutellidae) on head cabbage in the central rift valley of Ethiopia. Journal of Agricultural Research 4: 97-105.
- Bellati J, Mangano P, Umina P, Henry K (2012) Insects of Southern Australian Broadacre Farming Systems Identification Manual and Education Resource. Department of Agriculture and Food Western Australia (DAFWA), Australia.
- CABI (2017) Brassica oleracea var. capitata (cabbage) In: Invasive Species Compendium. Wallingford, UK.
- Carter CC, Sorensen KA (2013) Insect and related pests of vegetables. Cabbage and turnip aphid. Center for Integrated Pest Management. North Carolina State University, Raleigh, NC, USA.
- Carvalho LM, Bueno VH, Martinez RP (2002) Alate aphids survey on vegetable crops in Lavras (MG). Cien Agrotecnol 26: 523.
- Cervoni BR (2017) Cabbage Nutrition Facts, USA.
- CIMMYT (1988) Agronomic Data to Farmer Recommendations: An Economics Training Manual. Completely revised edition, Mexico.
- CSA (2015) Report of Federal Democratic Republic of Ethiopia, Statistical Report on Socio-Economic Characteristics of the Population in Agricultural Households, Land Use, Area and Production of Crops, Addis Ababa, Ethiopia.
- Duchovskien L, Raudonis L (2008) Seasonal abundance of Brevicoryne brassiae L. and Diaeretiella rapae (M’Intosh) under different cabbage growing systems. Ekologija 54: 260-4.
- Ellis PR, Kift NB, Pink DA, Jukes PL, Lynn J, et al. (2000) Variation in resistance to cabbage aphid (Brevicoryne brassicae) between and within wild and cultivated brassica species. Gene Resour Plant Evol 47: 395-401.
- Emana B, Gebremedhin H (2007) Constraints and opportunities of horticulture production and marketing in Eastern Ethiopia. Drylands Coordination Group, Norway.
- FAO (2012) Specifications and Evaluations for Agricultural Pesticides. Dimethoate O,O-dimethyl S-methylcarbamoylmethyl phosphorodithioate, 1-29.
- FAOSTAT (2007) Food and agriculture organization, United Nations, USA.
- Fening K, Ethelyn F, Francis W, Ibrahim A, Kwame A, et al. (2016) Aphids: A Major Threat to Cabbage Production in Ghana. Tropentag, Vienna, Austria.
- Fereres C, Gutierrez P, Del Estal, Castanera P (1988) Impact of the English grain aphid, Sitobion avenae (homoptera: Aphididae), on the yield of wheat plants subjected to water deficits. Environmental Entomology 17: 596-602.
- Gashawbeze A, Bayeh M, Mulugeta N, Yeshitila M, Lidet S, et al. (2009) Review of research on insect and mite pests of vegetable crops in Ethiopia.
- Getnet M, Raja N (2013) Impact of Vermicompost on Growth and Development of Cabbage, Brassica oleracea Linn. and their Sucking Pest, Brevicoryne brassicae Linn. (Homoptera: Aphididae),
- Gondar, Ethiopia. Research Journal of Environmental and Earth Sciences 5: 104-12.
- Gomez R (2011) Teaching the Analysis of Variance Using Technology Resources. Review of Higher Education and Self Learning. Florida International University 4: 1-6.
- Griffin RP, Williamson J (2012) Cabbage, Broccoli and Other Cole Crop Insect Pests. HGIC 2203, Home and Garden Information Center. Clemson Cooperative Extension. Clemson University, Clemson, SC, USA.
- Grzywacz D, Rossbach A, Rolf A, Russell DA, Srinivasan R, et al. (2010) Current control methods for Diamond back moth and other brassica insect pests in the prospect for improved management with lepidopteron resistant vegetable brasican in Asia and Africa, Africa.
- Habibullah B, Aminul I, Abdul M, Jashim U (2007) Effectiveness of Some Botanical Extracts on Aphids. Journal of Entomology 4: 136-42.
- Harsimran KG, Harsh G, Jennifer L, Gillet K (2016) Cabbage aphid Brevicoryne brassicae Linnaeus (Insecta: Hemiptera: Aphididae). Department of Entomology and Nematology, UF/IFAS Extension, USA.
- Hassan SA, Zhang WQ (2003) Use of the parasitoid Diaeretiella rapae (McIntoch) to control the cabbage aphid Brevicoryne brassicae (L.). J Appl Ent 127: 522-6.
- Hines RL, Hutchison WD (2013) Cabbage aphids on Vegetable IPM resource for the Midwest, University of Minnesota, Minneapolis, USA.
- IBM Corp (2011) IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp, USA.
- JahanF, Abbasipour H, Askarianzade A, Hasanshahi G, Saeedizadeh A (2013) Effect of eight cauliflower cultivars on biological parameters of the cabbage aphid, Brevicoryne brassicae (L.) (Hemipetera: Aphididae) in laboratory conditions. Archieves of Phytopathology and Plant Protection 46: 636-42.
- Kabir MM, Hossain MA, Farhat FS, Niaz M, Rahman F (2014) Effect of botanicals on aphid pest. International Journal of Innovative Research & Development 2278-311.
- Kalule T, Wright DJ (2002) Effect of cabbage cultivars with varying levels of resistance to aphids on performance of the parasitoid, Aphidius colemani (Hymenoptera: Braconidae). Bull Entomol Res 93: 53-9.
- Kazana E, Pope TW, Tibbles L, Bridges M, Pickett JA, et al. (2007) The cabbage aphid: a walking mustard oil bomb. Proc R Soc Lond Series B Biol Sci 274: 2271-7.\Kessing JM, Mau RL (1991) Cabbage aphid, Brevicoryne brassicae (Linnaeus). Crop Knowledge Master. Department of Entomology, Honolulu, Hawaii, USA.
- Khaliq M, Javed M, Sohail, Muhammad S (2014) Environmental effects on insects and their population dynamics. Journal of Entomology and Zoology Studies 2: 1-7.
- Khater HF (2011) Ecosmart Biorational Insecticides: Alternative Insect Control Strategies. In: Insecticides, Perveen, F. (Ed.). InTech, Rijeka, Croatia.
- Kibrom G, Kebede K, Weldehaweria G, Dejen G, Mekonen S, et al. (2012) Field evaluation of aqueous extract of Melia azedarach Linn. seeds against cabbage aphid, Brevicoryne brassicae Linn. (Homoptera: Aphididae), and its predator Coccinella septempunctata Linn. (Coleoptera: Coccinellidae). Archives of Phytopathology and Plant Protection 45: 1273-1279.
- Kidanie DG, Alemayehu M, Haileselassie A (2016) Assessment of production practices and effect of n: p2o5: s rates on yield and yield components of head cabbage (Brassica oleracea Var. Capitata) under irrigation conditions in Lay Armachiho district, Amhara Region, Ethiopa.
- Koona P, Njoya J (2004) Effectiveness of Soybean Oil and Powder from Leaves of Lantana camara Linn. (Verbenaceae) as Protectants of Stored Maize against Infestation by Sitophilus zeamais Motsch (Coleoptera: Curculionidae). Asia Network for Scientific Information, Asia.
- Koul VK (1998) Insect pest management on cauliflower (Brassica oleraca var. botryfis). HPKV Palampur 1012-103.
- Maribet LP, Aurea CR (2008) Insecticidal action of five plants against maize weevil, Sitophilus zeamais motsch (Coleoptera: Curculionidae). KMITL Sci Tech J Vol 8: 24-34.
- Megersa A (2016) Botanicals extracts for control of pea aphid (Acyrthosiphon pisum; Harris). Journal of Entomology and Zoology Studies 4: 623-7.
- Mekuaninte B, Yemataw A, Alemseged T, Nagappan R (2011) Efficacy of Melia azadarach and Mentha piperata plant extracts against cabbage aphid, Brevicoryne brassicae (Homoptera: Aphididae). World Applied Science Journal 12: 2150-4.
- Mengistie BT, Mol PJ, Peter O (2017) Pesticide use practices among smallholder vegetable farmers in Ethiopian Central Rift Valley. Environ Dev Sustain 19: 301-24.
- Michael AH, Raja N (2012) Evaluation of Melia azedarach Linn Croton macrostachys Hochst and Schinus molle Linn Plant Extracts against Cabbage Aphid Brevicoryne brassicae Linn and their Natural Enemies Diaeretiella rapae (Mintosh) and Hippodamia tredecimpunctata Lin. Ethiopia. Asian Journal of Agricultural Sciences 4: 411-8.
- Molla T, Asresie H, Biruhalem K, Baye B, Mekonen T, et al. (2005) Participatory Rural Appraisal Report: Dera District. Bahir Dar University CASCAPE.
- Munthali DC (2009) Evaluation of cabbage varieties for resistance to the cabbage aphid. African Entomology: 17: 1-7.
- Munthali DC, Tshegofatso AB (2014) Factors affecting abundance and damage caused by Cabbage Aphid, Brevicoryne brassicae on Four Brassica Leafy Vegetables: Brassica oleracea var. Entomology Journal 8: 1-9.
- Nagappan R (2012) Impact of Melia azedarach linn. (Meliaceae) dry fruit extract, farmyard manure and nitrogenous fertilizer application against cabbage aphid Brevicornye Brassicae linn. (Homoptera: Aphididae) in home garden, Ethiopia. Asian Journal of Agricultural Sciences 4: 193-7.
- Natwick ET (2009) Cole crops: cabbage aphid. UC Pest Management Guidelines. University of California Agriculture and Natural Resources, USA.
- Nayem Z, Rokib H (2013) Effects of Manually Processed Bio-pesticides on Crop Production and Pest Managements in Okra (Abelmoschus esculentus (L.)). Journal of Biopesticides 3: 7-8.
- Opfer P, McGrath D (2013) Oregon vegetables, cabbage aphid and green peach aphid. Department of Horticulture. Oregon State University, Corvallis, USA.
- Oyekale AS, Idjesa E (2009) Adoption of improved maize seed and production efficiency in River State, Nigeria. Acad J Plant Sci 2: 44-50.
- Pahla I, Moyo M, Muzemu S, Muziri T (2014) Evaluating the effectiveness of botanical sprays in controlling Aphids (Brevicoryne brassicae ) on cabbage. International Journal of Agronomy and Agricultural Research (IJAAR) 5: 1-6.
- Pakhraj P, Ganduli RN, Ganduli J, Dubey VK (2005) Pest succession in cabbage at Raipur, Chhattisgarh (India). J Appl Zoologic 16: 28-9.
- Pal M, Singh R (2013) Biology and ecology of the cabbage aphid, Brevicoryne brassicae (linn.) (Homoptera: Aphididae) : A review. The Aphidological Society. India Journal of Aphidology 27: 59-78.
- Pal M, Singh R (2012) Seasonal history of cabbage aphid, Brevicoryne brassicae (Linn.) (Homoptera: Aphididae). J Aphidol 25, 26: 69-74.
- Pontoppidan BO, Richard H, Rask L, Meijer J (2003) Infestation by cabbage aphid (Brevicoryne brassicae ) on oilseed rape (Brassica napus) causes a long lasting induction of the myrosinase system.
- Newzerlands entomology society, entomology experimentalists. Amersham biosciences 109: 55-62.
- Razaq M, Maqsood S, Aslam M, Shad SA, Afzal M (2012) Effect of plant spacing on aphid population, yield components and oil contents of late sown canola, Brassica napus L. (Brassicaceae). Pakistan Journal of Zoology 44: 991-5.
- Riddick EW (2017) Identification of Conditions for Successful Aphid Control by Ladybirds in Greenhouses. MDPI Insects 8: 38.
- Rohilla HR, Singh M, Kalra UK, Kharub SS (1987) Losses caused by cabbage aphid (Brevicoryne brassicae Linn) in different Brassica genotypes. Proceedings of 7th International Rapeseed Congress, Poland.
- Sarwar M (2015) The Killer Chemicals for Control of Agriculture Insect Pests: The Botanical Insecticides. Nuclear Institute for Agriculture and Biology, Faisalabad, Punjab, Pakistan. International Journal of Chemical and Biomolecular Science 1: 123-8.
- SAS Institute (2005) The SAS system for windows, version 9.0. SAS, Institute, Cary, NC, USA.
- Shiberu T, Negeri M (2016) Effects of Synthetic Insecticides and Crude Botanicals Extracts on Cabbage aphid, Brevicoryne brassicae (L.) (Hemiptera: Aphididae) on Cabbage. J Fertil Pestic 7: 162-4.
- Shoaib U (2003) Spatio-temporal distribution of aphid ((Brevicoryne brassicae L.) in Canola (Brassica napus L.). M.Sc. Thesis. University College of Agriculture, Bahauddin Zakariya University, Multan, USA.
- Singh CP, Sachan GC (2004) Assessment of yield losses in yellow sarson due to mustard aphid, Lipaphis erysimi (Kalt). Jouranl of Oilseeds Research 11: 179-84.
- Singh D, Prasad S, Singh R (2004) Demography of Diaeretiella rapae (McIntosh) (Hymenoptera : Braconidae, Aphidiinae), a parasitoid of Lipaphis erysimi (Kalt.) (Homoptera : Aphididae) reared on Brassica campestris Linn. J Aphidol 18: 65-70.
- South Gondar Administrative zone Agricultural Department Annual Report, 2012. Socio-economic and agricultural data of south Gondar administrative zone districts.
- Talekar NS (2000) Chinese cabbage. Proceedings of the 1st International Symposium on Chinese Cabbages. AVRDC, Shanhua, Tainan, Taiwan.
- Tesdeke A, Gashawbeza A (2014) Progress in vegetable management research proceedings of the National Horticultural Workshop, Addis Ababa, Ethiopia.
- Thompson JK (2002) Yield evaluation of cabbage varieties. J Agric Technol 5: 15-9.
- Umina P, Hangartner S (2015) Pests of field crops and pastures: pest notes. South Australian Research and Development Institute .CSIRO Publishing, Melbourne, Australia.
- Valenzuela I, Hoffmann A (2014) Effects of aphid feeding and associated virus injury on grain crops in Australia. Austral Entomology 10: 121-2.
- Vural HE¸ iyok D, Duman I (2000) The culture vegetables (vegetable growing). Izmir, Turkey.
- Wubie M, Negash A, Guadie F, Molla G, Kassaye K, et al. (2014) Repellent and Insecticidal Activity of Mentha piperita (L.) plant extracts against cabbage aphid [Brevicoryne brassicae Linn. (Homoptera: Aphididae)] American-Eurasian Journal of Scientific Research 9: 150-6.
- Yarou B, Assogba KF, Simon AC, Verheggen FJ, Francis F (20170 Efficacy of Basil-Cabbage Intercropping to Control Insect Pests in Benin, West Africa. Comm Appl Biol Sci Ghent University 82: 157.