Biology (SPBR)
Stechnolock: Plant Biology and Research
Full Text
Volume 1, Issue 1
GC-MS Analysis and Antifeedant Activity of Azaridiachta indica- Leaf Extract
*Corresponding Author: Sharma GD, Devi Ahilya Vishwa Vidhalaya, Indore, Madhya Pradesh, India, Tel: , E-mail: drgdsharma7@gmail.com
doi: /spbr.2021.1.107
Citation: Khanday S, Sharma GD (2021) GC-MS Analysis and Antifeedant Activity of Azaridiachta indica- Leaf Extract. Stechnolock Plant Biol Res 1:1-15
Copyright: © 2021 Sharma GD. This is an open-access article distributed under the terms of Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
In the present investigation, the feeding deterrent effects of methanolic and aqueous extracts of Azaridiachta indica by using leaf disc with no choice method with some modifications were assessed against cotton whitefly Bemesia tabaci. Five different concentrations ranging from 5% - 25 % of each extract were used and their antifeedant effect were recorded after different time periods (24, 48, and 72 hrs.) by comparing the averages of the leaf area consumed in the treated leaves and control leaves. The results clearly decipher that both extracts had antifeedant effects but comparing the extracts, the higher deterrent effect was attained by methanolic extract (87.37%± 12.07) at 25% concentration after 72 hours of post treatment. Antifeedant activity of solvent extracts was assessed based on antifeedant index. Higher antifeedant index normally indicates decreased rate of feeding. The methanolic leaf extract was more effective than that of aqueous solvent. The effect of the extracts was dose dependent and in positive correlation with the concentration. Furthermore, GC-MS based metabolic fingerprinting approach was also employed to find out the composition and relative abundance of active phyto-constituents. It was reported that the chromatogram of methanolic/aqueous extracts of azadirachta indica showed 91 and 88 peaks respectively, indicating more number of active constituents using methanol as extraction solvent. The main chemical constituents identified in this study may be responsible for the reported anti-feeding effect of the extract and could offer an alternative source of natural insecticide against Bemesia tabaci.
Keywords: Azadirachta indica; Deterrent effect; Bemesia tabaci; GC-MS analysis; Natural InsecticideIntroduction
The cotton whitefly Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) is a cosmopolitian, polyphagus and most serious agricultural cotton pest that has caused much heavy losses in productivity of crop, mainly in Fabaceae, Cucurbitaceae and Solanaceae (Oliveira et al. 2001) [1]. Bemisia tabaci is polyphagous inhabiting more than 600 host plant species. These major sucking insect pests are mainly managed by synthetic insecticides but due of their several adverse effects on environment and human health, plant derivative insecticides are being used by the farmers. At present, plant derived insecticides are well considered as one of the main convenient sources of biorational products with new modes of actions to control phytophagous insects (Rattan, 2010; Dayan et al., 2009) [2,3]. Currently more than 46 families of flowering plants are estimated that are known to possess insecticidal properties (Feinstein, 1952) [4]. Amongst these botanicals, neem tree is considered the most promising source for the management of these insect pests (Jacobson, 1988) [5]. It’s safe and ecofriendly nature makes it compatible for integrated pest management over the other synthetic insecticides. The control of B. tabaci has been taken by chemical insecticides, which dealt with high levels of resistance, damage to non-target organisms and environmental contamination (Elbert and Nauen, 2000) [6]. The hazardous impact of synthetic pesticides on human health and environmental encouraged the use of plant derived pesticides for insect pest management as they are non toxic, easily biodegradable and ecofriendly. A few literatures have dealt with the use of plant derived pesticides or their derivatives as potential bio-pesticides against whiteflies Bemesia tabaci. This study amied to screen out the phytochemical constituents in methanolic and aqueous leaf extracts of Azadirachta indica by GC-MS analysis and evaluate theantifeedant activity ofthese extracts against cotton white fly, Bemesia tabaci.
Materials and Methods
Collection of medicinal plant
Selected plant material i.e. leaves of Azadirachta indica were collected from different places of Indore region in poly bags and was identified and authenticated at centre for Biodiversity and Taxonomy, University of Kashmir under voucher no. 2248 KASH herb dated 2016. The leaves were shade dried, ground to powder and subjected to extraction in a Soxhlet extraction unit, using methanol and water as extraction solvents. The extraction was done at 30-45°C and finally the extracts were evaporated to dryness using a vacuum evaporator. The dry paste was stored in small vials at -80°C until further use.
Collection and rearing of insects
Adult whiteflies were collected from the cotton field. The stock of colony of Bemesia tabaci was maintained on cotton plants in entomological cages (1.2 x 1.2 x 1.0 m) under controlled conditions. The cages were kept in greenhouse at 25- 35ºC, 55-75% relative humidity and natural light (12:12h).
Antifeedant Bioassays Defago et al., (2006) [7]
The feeding deterrent effects of the Azadirachta indica extracts on Bemesia tabaci adults starved for 4–5 h prior to each bioassay was determined using leaf disc with no choice method with some modifications. Fresh cotton leaf discs of 1.5 cm in diameter were punched using a cork borer and methanolic and aqueous extracts were applied at different doses (5%, 10%, 15%, 20% and 25%) on both sides of leaf discs individually. Leaf discs treated with water were used as control. After air drying, each leaf disc was placed in petridish containing wet filter paper to avoid early drying of the leaf disc and 5 adults of Bemesia tabaci were introduced. For each concentration four replicates were maintained. All the experiments were carried under 18: 6 photoperiods at 20 °C. Bemesia tabaci adults were allowed to feed for about 12, 24 and 48 hours and the leaf discs were removed subsequently and the progressive consumption of the leaf disc area in all treatments was recorded using laser leaf area meter (CI- 203CA, CID Inc., WA). Leaf area eaten in each treatment group, was corrected by leaf area eaten in control. The percentage of antifeedant index was calculated using the formula of Jannet et al., (2000) [8].
Where AFI = Antifeedant Index;
C = Area protected in control leaf disc;
T = Area protected in treated leaf disc
Gas chromatography – Mass spectrometry (GC-MS) analysis
Metabolomic fingerprinting of methanolic and aqueous extracts of Azadirachta indica-leaves was carried out as described Roessner et al., (2000) [9] with some modifications. Both the alcoholic and aqueous extracts were dried and resuspended in methanol and filtered through 0.45µ syringe filter. About 2µl of each sample was injected in a GC-MS AP2010 Plus system (Shimadzu, Japan) equipped with a programmable head space auto injector/sampler and a Flame thermionic detector (FTD). The capillary used was DB- 1/RTXMS-1 (30 m) with helium gas as carrier at a constant flow rate of 1.21 ml/min. The samples were injected in a split less mode at an injection temperature of 260 °C/ column oven temperature of 60°C. The temperature gradient applied to GC oven, during the analysis was at 60 °C/ 2 minutes; then 250 °C at a rate of 5°C/minute for 2 minutes followed by a temperature ramp of 300 °C at a rate of 15 °C/minute for 15 minutes. The system was set at an ion source temperature of 220 °C with an interface temperature of 270 °C; detector gain volume at 0.00kV and the solvent cut time of 4.5 minutes in a relative gain mode. Mass spectra were recorded between 5.0-60.32 min. of injection in an ACQ scanning mode; event time of 0.5 sec/ scanning speed 1250 in the m/z range of 50-650. Identification of individual components was achieved by comparing the retention times and molecular masses of individual peaks from GC with those from the Wiley and National Institute of Standards and Technology (NIST) Library. The GC-MS was carried out at Advanced Instrumentation Research Facility (AIRF), Jawaharlal Nehru University (JNU), New Delhi.
Results
The results presented in Figures 1a and b indicate that both the methanolic and aqueous extracts of Azadirachta indica showed the antifeedent activity. Antifeedant property of solvent extracts was examined mainly by antifeedant index. Maximum antifeedant index revealed minimum amount of feeding. The maximum antifeedant activity at 72 hours of treatment was shown by methanolic extract 87.37%± 12.07 at 25% concentration while as at 5.0 % concentration the respective antifeedant value was 80.44%±13.21 at P<0.05. The corresponding antifeedant value of aqueous extracts at 25% concentration was 83.86±15.12. While at 5% concentration the respective antifeedant value was 74.93%±8.65 at P<0.05. Similarly, the maximum antifeedant activity at 48 hours of treatment was shown by methanolic extract 58.14%±7.43 at 25% concentration while as at 5% concentration the respective antifeedant value was 53.63%±7.23 at P<0.05. The corresponding antifeedant activities of respective aqueous extract at 25% concentration was 54.71%±6.23. While as 5% concentration the respective antifeedant values was 47.11%±7.21 at P<0.0. Similarly, the maximum antifeedant activity at 24 hours of treatment was shown by methanolic extract (40.90%±5.07) at 25% concentration while as at 5% concentration the respective antifeedant value was 36.67%±2.55 at P<0.05. The corresponding antifeedant activitiy of respective aqueous extract at 25% concentration was 39.69%±9.35. While as 5% concentration the respective antifeedant values was 30.74%±8.65 at P<0.05.
Above mentioned results clearly decipher that both the methanolic as well aqueous extracts of Azadirachta indica showed antifeedant activitiy at all concentrations and time durations of treatment, but comparing the extracts, methanolic extracts showed the maximum percentage of antifeedant activitiy at 25 % or 5% concentrations after 72 hours of post treatment while as aqueous extract showed the lowest percentage of antifeedant activitiy 25 % or 5% concentration after 24 hours of duration.
Thus the result illustrates that the antifeedant potential of extracts towards the pest was in a dose dependent manner-- the higher the concentration the greater the antifeedant activity and vice versa. However the effect seemed dependent on time of exposure as well.
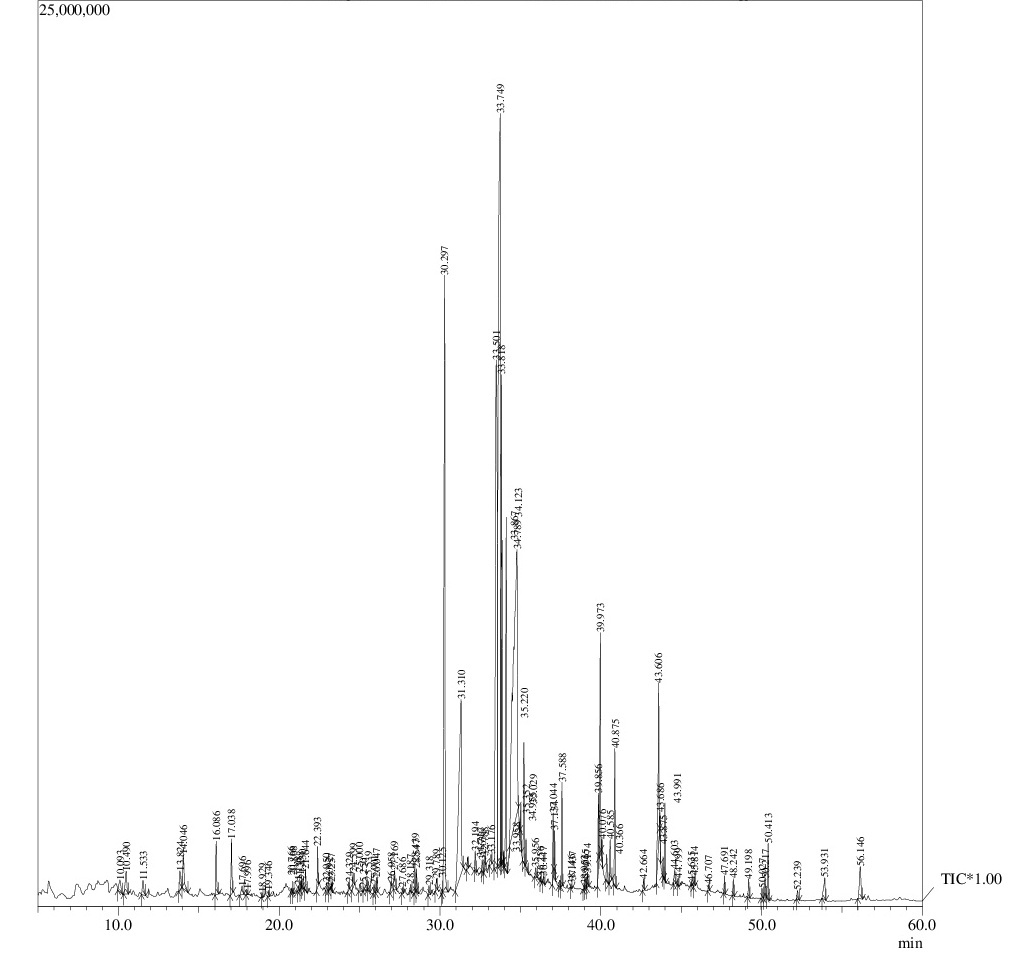
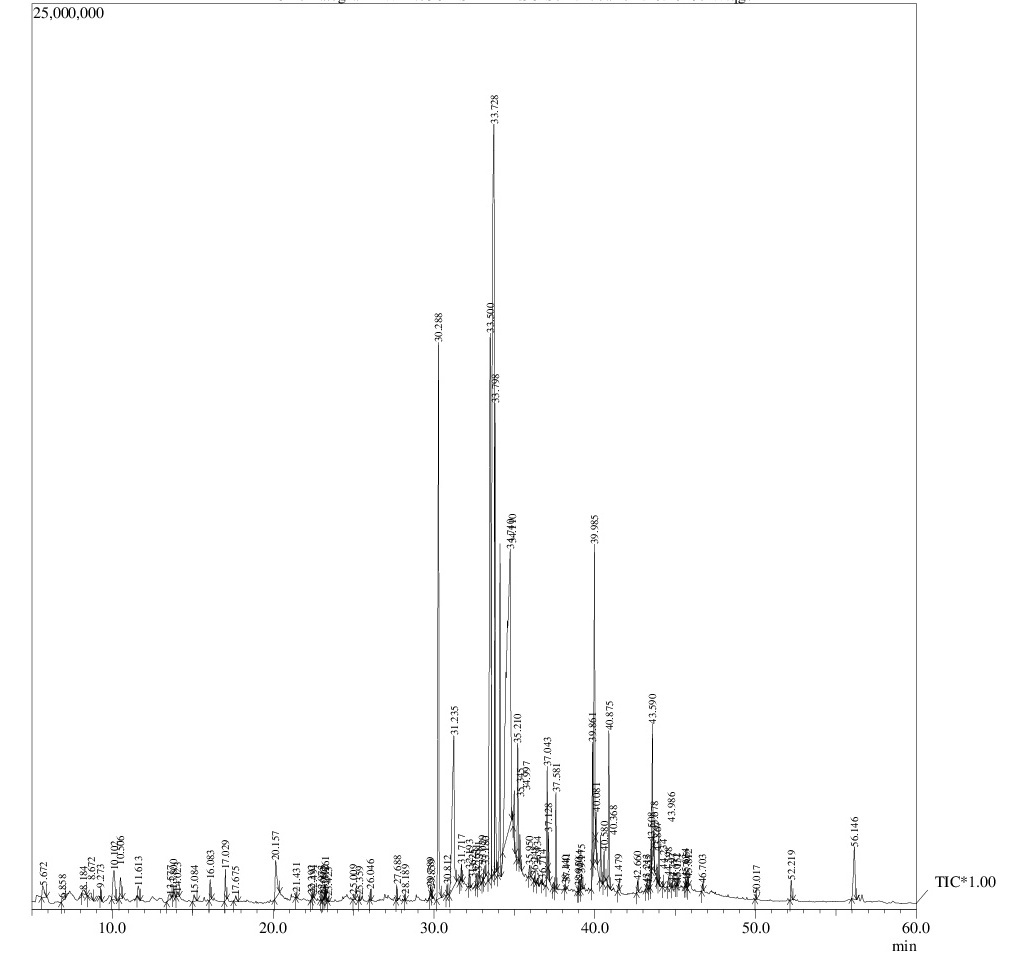
Gas chromatography- Mass spectrometry (GC-MS) analysis
To find out the composition and relative abundance of active phyto-constituents; using aqueous or alcoholic solvents and its correlation with the insecticidal activity, GC-MS based metabolic fingerprinting approach was employed. The chromatogram of methanolic/aqueous extracts of Azadirachta indica is represented in Figure 2a and b showing 91 and 88 peaks respectively, indicating more number of active constituents using methanol as extraction solvent. The constituents present in the methanolic/aqueous extracts of Azadirachta indica, corresponding to the chromatogram peaks along with their retention time (RT), percent peak area and the identified name from NIST- WILEY library are shown in Table 1a and b. It is clear from the table that the most abundant constituents- 15 & 12 from each extract (in terms of percent peak area) lie in the range of 1-25%, constituting 87.60% and 83.04% of total percent peak area of aqueous and methanolic extracts, respectively. To compare the insecticidal activity and hence efficiency of two extraction solvents, it was further analyzed the abundance of component/s in different class intervals of percent peak areas. The biological activity of each predominant compound is also shown (Table 2a and b), reflecting their bioactivities and benefits.
Discussion
Azadirachta indica derivatives showed more reduction of the insect pest population. This is mostly due its deterrent and antifeedant effect which compell whiteflies to fly away from that locality. Khattak et al., (2000) [10] investigated that the detrimental effect of 1000ppm neem oil treatment lost by 30 days after treatment but the 10,000ppm treatment effectively retained its antifeedant and deterrent effects against maize weevil on corn kernels. Khan et al., (2002) [11] also demonstrated that due to the antifeedant and deterrent effect of Azadirachta indica extracts, the populations of jassids, thrips and whiteflies on cotton significantly reduced 17 days after spray.. Silva (2007) [12] investigated the antifeedant properties of the hydroalcoholic extract obtained from the leaves of Azadirachta indica on Zabrotes fasciatus (Coleoptera: Bruchidae), an insect pest that commonly feeds on common bean (Phaseolus vulgaris) during seed storage and observed the significant antifeedant activity when it was added to the insect diet. Alice Sujeetha (2008) [13] showed that on rice, extracts of neem seeds and neem leaves inhibit the growth and development of Sogatella furcifera (Horvath) (Homoptera: Delphacidae). These results are in agreement with the present investigation.
Previously the ethyl acetate extract of leaf of Azadirachta indica has been shown to contain a total of 30 volatile compounds with Hexadecanoic acid; 9/10 Octadecanoic acid methyl ester; methyl stearate; cis 11- Eicosanoic acid; Docasonoic acid methyl ester as major constituents Praveen et al., (2018) [14]. In compliance to this report, our study reports a total of 91 and 88 peaks from methanolic and aqueous extracts of neem leaf respectively, corresponding to 12 and 15 major constituents/ peaks. In addition to this, out of total 30 peaks from fruit sap/pulp of neem, Hexadecanoic and Pentadecanoic acids were the major peaks/ fatty acids Kumar et al., (2018) [15]. Further a study reported only 4 peaks from methanolic fraction of neem leaf comprising- m-Toluyl-aldehyde; Methyl 14-methylpentadecanoate; Linoleic acid chloride and Methyl isoheptadecanoate while they were comparing different solvent systems with the richness of chromatogram produced Hossain et al., (2013) [16].
To sum up, it is quite evident that the approach of selection of water and methanol as extraction solvents yielded a significant higher proportion of bioactive components comprising predominantly of fatty acids or their esters with diversifying activities. However, to validate the lead insecticidal molecules further studies are needed to perform bioactivity based fractionation as well as characterization of active molecules. This could help to synthesize the active lead insecticidal molecules in the lab with better efficacies and least side effects thereby preventing the plant wealth and to maintain nature’s diversity without disturbing the ecological balance of the planet [17,31].
Conclusion
According to the results obtained in the current study, it can be concluded that both methanolic and aqueous extracts of Azadirachta indica presented a high insecticidal activity against B. tabaci and showed positive relationship with concentration. The results of this study raises the possibility that the insecticidal properties of the active compound(s) present in the tested plant extracts could be exploited as an alternate of many synthetic chemical insecticides being indiscriminately used for control of B. tabaci.
Conflicts of interest
We declare no conflict/s of interest related to this work.
Acknowledgement
We declare that since the work was not supported by any kind of funding from any source, so sincerely we wouldn’t acknowledge anyone for financially supporting the work. However, the corresponding author sincerely acknowledges all the authors for the successful completion of the work in the present form.
- Vanlauwe B, Bationo A, Chianu J, Giller KE, Merckx R, et al. (2010) Integrated soil fertility management: operational definition and consequences for implementation and dissemination. Outlook on Agric 39: 17-24.
- Zamir MSI, Yasin G, Javeed HMR, Ahmad AUH, Tanveer A, et al. (2013) Effect of different sowing techniques and mulches on the growth and yield behavior of spring planted maize (Zea may L.). Cercetări Agronomic in Moldova, 1: 77-82.
- Zeinab AB, Hossein Z, Masoud R (2014) Effect of Vermicompost andChemical Fertilizers on Growth Parameters of three Corn Cultivars. J Appl Sci Agric 9: 22-6.
- Abera Y and T Belachew (2011) Local perceptions of soil fertility management in southeastern Ethiopia. Int Res J Agric Sci 1: 64-9.
- Wakene N, and Heluf G (2004) The Impact of Different Land Use Systems on Soil Quality of Western Ethiopian Alfisols, International Research Food Security: Natural Resource Management and Rural Poverty Reduction through Research for Development and Transformation, Deutcher Tropentage-Berlin 1-7.
- Yadav A and Garg VK (2011) Recycling of organic wastes by employing, Eisenia fetida. Bioresource Technology, 102: 2874–80.
- Alam MN, Jahan MS, Ali MK, Ashraf MA and Islam MK (2014) Effects of vermicompost and chemical fertilizers on growth, yield and yield components of potato in barind soils of Bangladesh, Journal Applied Science Research, 3: 1879-88.
- Tolera A, Tolcha T, Tesfaye M, Haji K and Buzuayehu T (2018) Effect of Integrated Inorganic and Organic Fertilizers on Yield and Yield Components of Barley in Liben Jawi District. International Journal of Agronomy, 10: 1-7.
- Tariku B, Tolera A and Ermiyas H (2018) Effect of Integrated Nutrient Management on Growth and Yield of Food Barley (Hordeum vulgare L.) Variety in Toke Kutaye District, West Showa Zone, Ethiopia. Advances in Crop Science and Technology, 6: 1-8.
- Abera W, Hussein S, Derera M, Worku D (2013) Preferences and constraints of maize farmers in the development and adoption of improved varieties in the mid-altitude, sub-humid agro-ecology of Western Ethiopia. African Journal of Agricultural Research, 8: 1245-54.
- Lazcano C, Gómez-Brandón M and Domínguez (2008) Comparison of the effectiveness of composting and Vermicomposting for the biological stabilization of cattle manure. Chemosphere, 72: 1013-9.
- Suparno P, Talkah B and Soemarno A (2013) Applicationof vermicompost on organic mustard farming in Kediri. Indonesian Green Technology Journal 2: 78-83 (in Indonesian).
- Ahmadabadi Z, Ghajarspanlo M and Rahimialashti S (2011) Effect of vermicompost on soil chemical and physical properties, Science and Technology of Agriculture Natural Resources. Soil and Water Sci 58: 125-37.
- Fekadu S, Brandon MG, Franke IH, Praehauser B, Insam H, et al. (2014) Coffee husk composting: An Investigation of the Process Using Molecular and Non-Molecular Tools. Waste Management, 34: 642-52.
- ATA (Agricultural Transformation Agency). 2014. Soil fertility status and fertilizer recommendation atlas for Tigray regional state, Ethiopia. MOA; ATA, Addis Abeba.
- ATA (AgriculturalTechnology Agency). 2009. Directory of Released Crop Varieties Addis Ababa, Ethiopia
- Arif, MI, Amin MT, Jan I, Munir K, Nawab NU, et al. (2010) Effect of Plant Population, Nitrogen Levels and Methods of Application on Ear Characters and Yield of Maize.
- Sahlemedhin S and Taye B (2007) Procedure for soil and plant analysis Technical Bulletin No.74.National Soil Research Center, Ethiopian Agricultural Organization, Addis Ababa, Ethiopia.
- Day PR (1965) Particle Fraction and Particle Size Analysis Methods of Soil Analysis Black, C.A. (Ed.) 9. Am Soc Agro 20: 545-67
- Soil Survey Staff. 1999. Soil Taxonomy. A basic system of soil classification for making and interpreting soil surveys. Second edition. Agriculture Handbook 436.Washington, DC, USDA. p886.
- Jamison VC, Weaver HH and Reed IF (1950) A hammer-driven soil core sampler. Soil Science, 69: 487-96.
- Rao M, Singa P and Raju MJ (2005) Laboratory Manual on Soil Physicochemical Properties. Aditha Art Printers, New Delhi, India.
- Rowell DL (1994) Soil Science: Methods and Applications. Longman and Scientific Technical, Harlow UK., ISBN-13: 9780470221419, Pages: 350
- Reynolds SG (1970) The gravimetric method of soil moisture determination part I: a study of equipment, and methodological problems. J of Hydrol 11: 258-73.
- Peech M (1965) Hydrogen-ion activity. pp. 914-26. In: CA, Black, D.D. Evans, J.L. Ensminger and F.E. Clark (eds). Methods of Soil Analysis. Part II.ASA, WI, Madison, USA.
- Walkly A and Black IA (1934) An Examination of digestion method for determining soil organic matter and a proposed modification of the chromic acid titration. Soil Sci 37: 29-38.
- Nelson DW and Sommers LE (1996) Total Carbon, Organic Carbon, and Organic Matter, In: Sparks, D.L., Eds., Methods of Soil Analysis, Part 3, Chemical Methods, SSSA Book Series No. 5, SSSA and ASA, Madison, WI, 961-1010
- Jackson ML (1958) Soil Chemical Analysis. Prentice Hall, Inc., Englewood Cliffs. New Jersey
- Bray RH and Kurtz LT (1945) Determination of total organic and available forms of phosphorus in soils. Soil Science, 59: 39-46.
- Murphy J and Riley JP (1962) A modified single solution method for the determination of phosphorus in natural waters. Analytica Chimica Acta, 27: 31-6.
- Warman PR and Sampson HG (1992) Evaluation of soil sulfate extractants and method of analysis for plant available sulfur. Communication in Soil Science and Plant Analysis, 7: 793-803.
- Jackson ML (1973) Soil Chemical Analysis, Prentice Hall India Pvt Ltd, New Delhi, India, 498-516.
- Hesse PR (1972) A text book of soil chemical analysis, John Murray Limited, London, Great Britain. 470p
- USDA (United State Department of agriculture) .2017. GAIN Report on Ethiopia (report No ET1634). Global Agricultural Information Network.
- Chapman H and Pratt (1961) Methods of analysis for soils, plant and water. University California, Berkeley.
- Bremner JM and Mulvaney CS (1982) Nitrogen total 1. Methods of soil analysis. Part 2. Chemical and microbiological properties,
(methods of soil an2) 595-624. - Olsen SR, Cole CV, Watanabe FS and Dean LA (1954) Estimation of Available Phosphorus in Soil Extraction with Sodium Bicarbonate, USDA Circ.939, U.S. Government Printing Office, Washington DC
- Hesse PR, and Hesse PR (1971) A textbook of soil chemical analysis
- SAS (Statistical Analysis Software).2004. SAS Software Syntax, Version 9.0, SAS Institute, Cary, NC, USA.
- Okoko ENK and Makworo S (2012) Evaluation of the effect of compost and inorganic fertilizer on maize yield in Nyamira District, Southwest Kenya, Kenya Agricultural Research Institute, Regional Research Centre
- Hazelton P and Murphy B (2007) Interpreting soil test results, what do all the numbers mean. Victoria, CSIRO Publishing.
- Negessa G and Tesfaye W (2021) Influence of Organic and Chemical Source Fertilizers on Soil Physicochemical Properties and Nutrient Concentration of Nitisol in Welmera District, Central Ethiopia. World Journal of Agricultural Sciences, 17: 295-307.
- Negassa W, Abera T, Minale Liben TD, Workayehu T, Menna A, et al. (2012) Soil Fertility Management Technologies for Sustainable Maize Production in Ethiopia, In Meeting the Challenges of Global Climate Change and Food Security through Innovative Maize Research, page 123).
- Landon JR (1991) Booker Tropical Soil Manual: A Hand Book for Soil Survey and Agricultural Land Evaluation in the Tropics and
Subtropics. Booker Tate limited, London, England. - Papini R, Valboa G, Favilli FL, abate G (2011) Influence of land use on organic carbon pool and chemical properties of Vertic Cambisols in central and southern Italy, Agricultural Ecosystem Environ.140: 68-79
- Pandey KK and Awasthi A (2014) Integrated nutrient management in the maize (Zea mays L.) yield and soil properties. Int J of
Agric Sci 10: 244-2 - Berhanu, D (1980) The physical criteria and their rating proposed for land evaluation in the highland region of Ethiopia. Land
Use Planning and Regulatory Department, Ministry of Agriculture, Addis Ababa, Ethiopia. - Tekalign T, Haque I and Aduayi EA (1991) Soil, plant, water, fertilizer, animal manure and compost analysis manual. Plant Science Division working document, ILCA, Addis Ababa, Ethiopia.13: 103 pp.
- Tesfaye W, Kibebew K, Bobe B, Melesse T and Teklu E (2018) Long Term Effects of Cultivation on Physicochemical Properties of
Soils at Metahara Sugar Estate. American- Eurasian Journal of Agricultural Research, 18: 246-57. - Tekalign M and Haque I (1991a) Phosphorus status of some Ethiopian soils. III. Evaluation of some soil test methods for available
phosphorus. Tropical Agriculture, 68: 51-6. - Jones JB (2003) Agronomic Handbook: Management of Crops, Soils, and Their Fertility. CRC Press LLC, Boca Raton, FL, USA.
482p. - Priya S, Kaushik MK, Sharma SK, and Priyanka K (2014) Impact of integrated nutrient managemnet on growth and productivity of hybrid maize (Zea mays L.), Annals of Biol 30: 106-8.
- Raman S (2018) Effect of integrated nutrient management on the growth and yield of hybrid maize, Journal of Agricultural Research, 3: 1-4.
- Tekalign M and Haque I (1991b) Phosphorus status of some Ethiopian soils, II.Forms and distribution of inorganic phosphates and their relation to available phosphorus. Tropical Agriculture, 68: 2-8
- Tilahun G (2007) Soil fertility status as influenced by different land uses in Maybar areas of South Wello Zone, North Ethiopia.
MSc. Thesis, Haramaya University, Haramaya, Ethiopia.40. - Brady N and Weil R (2008) The nature and properties of soils, 14th edition. Prentice Hall, Upper Saddle River. 992.
- Demir Z, and Gulser C (2015) Effects of rice husk compost application on soil quality parameters in greenhouse conditions.
European Journal of Soil Science 4: 185-90 - Tesfaye W, Kibebew K, Bobe B, Melesse T and Teklu E (2019) Effects of Subsoiling and Organic Amendments on Selected Soil Physicochemical Properties and Sugar Yield in Metahara Sugar Estate. American- Eurasian J Agric Res 19: 312-25.
- Hillel D (1998) Environmental Soil Physics. Academic Press, London, England
- Sanginga N and Woomer PL (2009) Integrated Soil Fertility Management in Africa: Principles, Practices and Development Process, CIAT, Nairobi, ISBN: 978929059261, Pages: 263
- Mirzaei K, Sabahi H and Damghani A (2009) Application of organic fertilizers and soil physical and chemical properties and dry matter production of tomato, J of Agric Res 7: 257-26.
- Murphy HF (1968) A report on fertility status and other data on some soils of Ethiopia. Collage of Agriculture HSIU. Experimental Station Bulletin No. 44, Collage of Agriculture: 551p.
- Tesfaye W, Kibebew K, Bobe B, Melesse T and Teklu E (2020) Effects of long term sugarcane production on soils physicochemical properties at Finchaa sugar Estate. J of Soil Sci and Environ Manage 11: 30-40.
- Tekalign T (1991) Soil, plant, water, fertilizer, animal manure andcompost analysis.Working document No. 13.International
LivestockResearch center for Africa, Addis Ababa. - Angelova VR, Akova VI, Artinova NS and Ivanov K (2013) The effect of organic amendments on soil chemical characteristics.
Bulgarian Journal of Agricultural Science 19: 958-71 - Zupanc V, and Zupanc JM (2010) Changes in soil characteristics during landfill leach ate irrigation of populous deltoids. Waste Management, 30: 2130-36.
- Landon JR (2014) Booker Tropical Soil Manual: A Hand Book for Soil Survey and Agricultural Land Evaluation in the Tropics and
Subtropics. Booker Tate limited, London, England. - Sharma UC and Singh K (1991) Integrated management of phosphate and farmyard manure in potato-radish cropping sequence on an acidic soil. J of Indian Soc Soil Sci 39: 468-47.
- Inal A, Günes A, Alpaslan M, Sait Adak M, Taban S, et al. (2003) Diagnosis ofsulfur deficiency and effects of sulfur on yield and yield components of wheat grownin Central Anatolia. Turkey J of Plant Nutr 26: 1483-98
- Habtamu A, Heluf G, Bobe B and Enyew A (2015) Effects of organic and inorganic fertilizers on yield and yield components of maize at Wujiraba Watershed, Northwestern Highlands of Ethiopia. Am J of Plant Nutr and Fertilization Technol 5: 1-15.
- Kebede F and Yamoah C (2009) Soil fertility status and numass fertilizer recommendation of typic hapluusterts in the Northern highlands of Ethiopia. World Applied Sci J 6: 1473-80.
Kibunja CN, Mwaura FB, and Mugendi DN (2010) Long-term land management effects on soil properties and microbial populations in a maize-bean rotation at Kabete, Kenya. Afr J of Agric Res 5: 108-13. - Karmakar S, Gangopadhyay A, Brahmachari K and Bandyopadyay PK (2009) Soil Health Management by applying Vermicompost prepared from Organic Wastes. J of Interacademicia, 13: 412-7
- FAO (Food and Agriculture Organization). 2006. Plant nutrition for food security: A guide for integrated nutrient management. FAO, Fertilizer and Plant Nutrition Bulletin 16, Rome.
- Ibrahim MM, Mahmoud EK, Ibrahim DA (2015) Effects of vermicompost and water treatment residuals on soil physical properties and wheat yield. Int Agrophys (Vc):157-164
- Brar BS, Singh K, Dheri GS, Kumar B (2013) Carbon sequestration and soil carbon pools in a rice–wheat cropping system: Effect of long term use of inorganic fertilizers and organic manure. Soil Tillage Res 128: 30-6.
- Ngome AFE, Becker M and Mtei KM (2011) Leguminous cover crops differentially affect maize yields in three contrasting soil
types of Kakamega, Western Kenya. Journal of Agriculture and Rural Development in the Tropics and Subtropics (JARTS), 112: 1-10. - Mitiku W and Asnakech H (2016) Effect of nitrogen fertilizer on growth, yield and yield components of maize (Zea mays L.) in decha district, southwestern Ethiopia. International Journal of Research- Granthaakayah, 4: 95-100.
- Dagne C (2016) Blended Fertilizers Effects on Maize Yield and Yield Components of Western Oromia, Ethiopia. Agriculture, Forestry and Fisheries 5: 151-62.
- Opala PA, Kisinyo PO, Nyambati RO (2015) Effects of farmyardmanure, urea and phosphate fertilizer application methods on maizeyields in western Kenya. J. Agric. Rural Dev. Trop. Subtrop, 116: 1-9.
- Pisa C, Wuta M (2013) Evaluation of composting performance of mixtures of chicken blood and maize stover in Harare, Zimbabwe. Int.J. Recycl. Organic Waste Agric. 2: 1-11.
- Oliveira MR, Henneberry TJ, Anderson P, (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Protection 20: 709-23.
- Rattan RS (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Protection 29: 913-92.
- Dayan FE, Cantrell CL, Duke SO (2009) Natural products in crop protection. Bioorganic & Medicinal Chemistry 17: 4022-34.
- Feinstein L (1952) Insecticides from Plants. Insects (The Year Book). United States Department of Agriculture, Washington D. C.: 222-9.
- Jacobson M (1988) Focus on phyto-chemical pesticides. Vol:1. The Neem tree. CRC Inc. Boca Raton, Florida, USA.
- Elbert A, Nauen R (2000) Resistance of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides in southern Spain with special reference to neonicotinoids. Pest Management Science 56: 60-4.
- Defago M, Valladares G, Banchio E, Carpinella C, Palacios S (2006) Insecticide and antifeedant activity of different plant parts of Melia azedarach on Xanthogaleruca luteola. Fitoterapia 77: 500-5.
- Jannet BH, Skhiri H, Mighri Z, Simmonds M, Blaney W (2000) Responses of Spodoptera littoralis larvae to Tunisian plant extracts and to neo-clerodane diterpenoids isolated from Ajuga pseudoiva leaves. Fitoterapia 71: 105-12.
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J 23: 131-42.
- Khattak MK, Broce AB, Dover B (2000) Egg developmental inhibition and ovipositional deterrence of Neem or Mineral oil on Maize weevil, Sitophillus zeamais Motsch. Pakistan Journal of Biological Sciences 3: 1910-3.
- Khan L, Khattak MK, Awan MN, Hussain AS (2002) Comparative efficacy of neem (Azadirachta indica. A. Juss) extracts and certain insecticidal combinations against thrips and boll worms on cotton. Gomal University Journal of Research 19: 251-8.
- Silva DB, Matos MFC, Nakashita ST, Misu CK, Yoshida NC, et al. (2007) Isolamento e avaliação da atividade citotóxica de alguns alcalóides oxaporfínicos obtidos de Annonaceae. Química Nova 30: 1809-12.
- Alice Sujeetha RP (2008) The biological and behavioral impact of some indigenous plant products on rice white backed plant hopper (WBPH) Sogatella furcifera (Horvath) (Homoptera: Delphacidae). Journal of Biopesticides 1: 193-6.
- Praveen K (2018) Analysis of volatile compounds in the sap of Azadirachta indica (neem) using gas chromatography mass spectrometry. Int Res J Pharm 9: 38-43.
- Kumar P, Nandhini AR, Subhapriya P, Gowrishankar BA (2018) Analysis of volatile compounds in the sap of Azaridiachta indica (neem) using gas chromatography mass spectrometry. International Journal Research of Pharmacy 9: 38-43.
- Hossain MA, Al-Toubi W, Weli A, Al-Riyami Q, Al-Sabahi J (2013) Identification and characterization of chemical compounds in different crude extracts from leaves of Omani neem. Journal of Taibah University for Science 7: 1-10
- Aleryani SL, Cluette-Brown JE, Khan ZA, Hasaba H, Lopez de Heredia L, et al. (2005) Fatty acid methyl esters are detectable in17 the plasma and their presence correlates with liver dysfunction. Clinica Chimica Acta 359: 141-9.
- Biljana BP (2012) Historical reviews of medicinal plants usage. Pharmacognosy Review 6: 1-5.
- Bhute NK, Bhosle BB, Bhede BV, More DG (2012) Population dynamics of major sucking pests of Bt cotton. Indian Journal of Entomology 4: 246-52.
- Devi N, Prabakaran J, Wahab F (2012) Phytochemical analysis and enzyme analysis of endophytic fungi from Centella asiatica. Asian Pacific Journal of Tropical Biomedicine 1280-4.
- Duke JA (2013) Beckstrom-Sternberg SM, Phytochemical and Ethnobotanical Databases, Duke’s Phytochemical and Ethnobotanical Databases, Europe.
- Galli C, Calder PC (2009) Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Annals of Nutrition and Metabolism 55: 123-39.
- Gehan MA, Hanan AE, Hassan AH, Okbah MA (2009) Marine natural products and their potential applications as anti-infective agents. World Sci J 7: 872-80.
- Gopalakrishnan, Kalaiarasi (2013) Comparative DFT Study of Phytochemical Constituents of the Fruits of Cucumis trigonus Roxb. and Cucumis sativus Linn. International Journal of Biological pharmaceutical Research 4: 523-7.
- Kapoor R, Huang YS (2006) Gamma linolenic acid: An antiinflammatory omega-6 fatty acid. Current Pharmaceutical Biotechnology 7: 531-4.
- Kuppuswamy KM, Jonnalagadda B, Arockiasamy S (2013) GC-MS analysis of chloroform extract of croton bonplandianum. International Journal of Pharma and Bio Sciences 4: 613-7.
- Lima B, Lopez S, Luna L, Aguero MB , Aragon L, et al. (2011) Essential oils of medicinal plants from the Central Andes of Argentina: chemical composition, and antifungal, antibacterial, and insect-repellent activities. Chemistry & Biodiversity 8: 924-36.
- Mathew OP (2011) Apnea of prematurity: Pathogenesis and management strategies. Journal of Perinatology 31: 302-10.
- Rani S, Mohan VR, Regini GS, Kalidass C (2009) GC-MS analysis of ethanolic extract of Pothos scandens leaf. Journal of Herbal Medicine and Toxicology 3: 159-60.
- Sivakumar RA, Jebanesan M, Govindarajan, Rajasekar P (2011) Oviposition attractancy of dodecanoic, hexadecanoic and tetradecanoic acids against Aedes aegypti and Culex quinquefasciatus. Asian Pacific Journal of Tropical Medicine 4: 706-10.
- Willits CO, Ricciuti C, Knight HB, Swern D (1952) Polarographic Studies of Oxygen-containing Organic Compounds. Analytical Chemistry 24: 785-90.